- January 14, 2020
- clarifiasd
- No Comments
- science
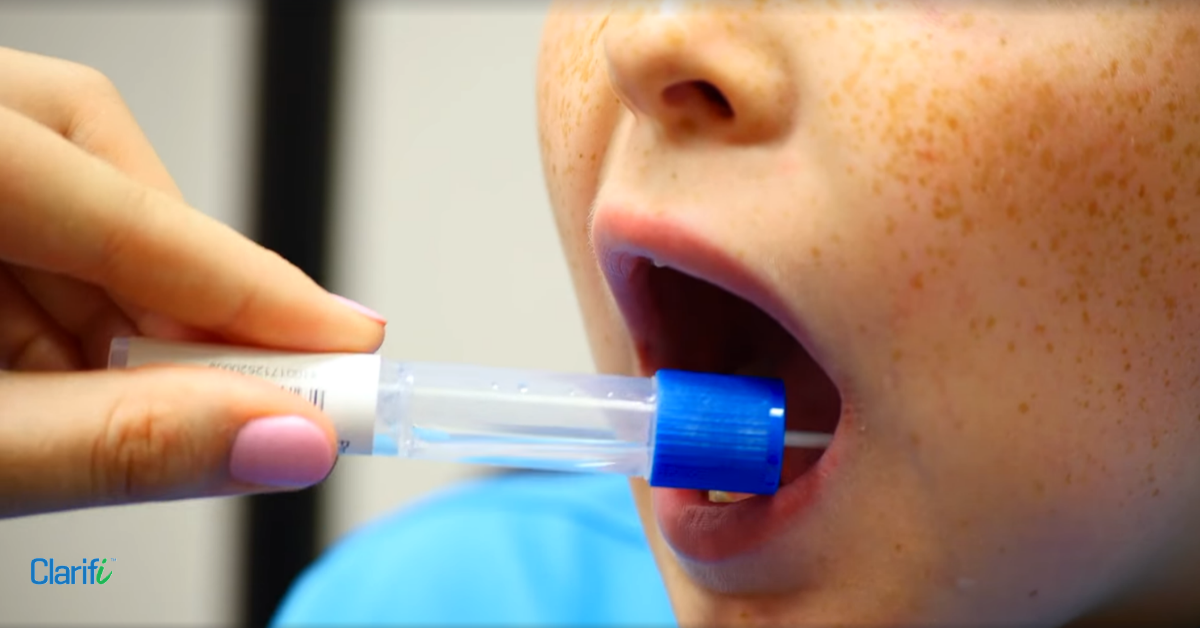
Introducing The Clarifi ASD Autism Saliva Test
The Clarifi ASD saliva test for autism is now available
The Clarifi ASD autism saliva test is a clinically-validated test designed to provide a probability of an autism diagnosis to health care providers. With a simple saliva swab, Clarifi ASD can provide children with a clinical suspicion of autism, such as a positive screen on the MCHAT-R, biological support for an earlier autism diagnosis.
Watch | Dr. Frank Middleton introduces Clarifi ASD
This groundbreaking test is based on years of research on epigenetic biomarkers in the saliva led by Dr. Frank Middleton, Ph.D., of SUNY Upstate Medical University. In 2018, he and his colleagues published “Validation of a Salivary RNA Test for Childhood Autism Spectrum Disorder” in Frontiers in Genetics.
“What we’ve been able to do…is develop a test that utilizes saliva and can accurately distinguish whether a child has autism spectrum disorder or does not,” Middleton explains. “This relies on a new type of molecular analysis that wasn’t even possible until recently.”
This new molecular analysis is what sets Clarifi ASD apart from other tests on the market. Clarifi ASD is an epigenetic test, meaning it looks at RNA rather than DNA. Using next-generation sequencing, a child’s saliva is analyzed for specific RNA molecules and bacteria from the microbiome to determine the probability of that child having autism.
According to the company, “Clarifi harnesses new RNA molecules that most scientists didn’t even know existed ten years ago. These molecules are critical for brain development, and what’s more, they’re present in high levels in the saliva. This allows us to do a non-invasive test at an outpatient pediatrics office and identify changes in the levels of molecules that differentiate kids with autism from kids who have typical development.”
Clarifi ASD is validated for children 18 months through 6 years old. This epigenetic test must be ordered and administered by a healthcare provider and is currently available in 49 states in the US. Clarifi ASD represents a new approach to aid in the diagnosis of autism, so it is not yet covered by insurance. However, it is currently being offered at an introductory price of $989, a significant discount relative to other tests using next-generation sequencing technology.
Clarifi ASD can be administered in-clinic at an appointment with a child’s regular physician.
After the patient has been swabbed, the sample is sent to a CLIA-certified lab for analysis, with results sent to the ordering healthcare provider within three to six weeks. Parents are encouraged to schedule a followup appointment with their healthcare provider to go over the results.
For more information about Clarifi ASD, including published papers, videos, and how to get the test, visit ClarifiASD.com

